ラブローションを取り巻く規制
性交時に使用するための潤滑剤というものがあります。
薬局や量販店などでは、コンドームの近くにそっと置かれていることが多いです。
潤滑ゼリー、潤滑ローション、ラブローション、ルブリカントなどとも呼ばれているようです。
今回は、この「性交時に使用する潤滑剤」にスポットを当てて、規制周りを見てみたいと思います。
性交時に使用する潤滑剤にかかる規制
正直、行政の手があまり入っておらず、多くの製品たちがグレーゾーンの混沌の中で蠢いている、という印象です…。
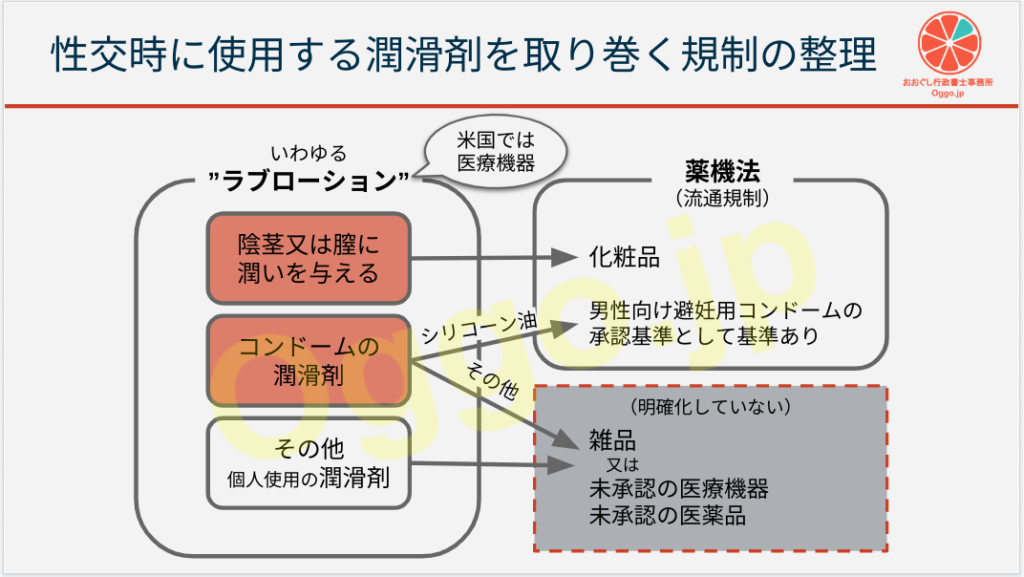
大まかな理解ではありますが
- 滑りを良くすることのみを目的としているならば、雑品
- 人体・皮膚への効果がある又は訴求しており、それが化粧品の範囲内(潤いを与えるとか)ならば化粧品、範囲内に収まらないならば医薬品
というところに現状収まるかなと思います。
※実際には自己判断せず、製品ごとに照会等を行い、区分を確実にすることをおすすめします。
規制の今後について
現在確認できる以下の事実があります。
- FDAは、米国内の潤滑剤について、クラスⅡの医療機器と分類。510(k)の認可を要求している(参考1及び参考2)。
それらの定義には「intended to moisturize and lubricate, to enhance the ease and comfort of intimate sexual activity, and supplement the body's natural lubrication.(Google翻訳:保湿および潤滑を行い、親密な性行為の容易さと快適さを高め、身体の自然な潤滑を補うことを目的とする)」が含まれる。 - 日本でも既に「歯科用潤滑材」という医療機器が存在する(参考3)。
その定義は「義歯と口腔粘膜との間の潤滑不足による不快感を抑制するために、義歯床、人工歯又は口腔粘膜表面に塗布して潤滑性を付与する材料をいう。医薬品及び生物由来材料を含むものを除く。」である。
個人的な意見としては、性交時に使用する潤滑剤は、粘膜部に使用するという性質上、品質・安全性の担保が必要で、それは雑品では難しいだろうと考えます。
セクシャルウェルネスを考える上で重要な製品だと考えますので、日本でも今後きちんと整理されていくことを期待しています。
なお、既製品のなかには「舐められる」「口に入っても安全」といった訴求がされている場合が見受けられました。
食品として流通させているわけでは当然ないので、不適切な広告だと考えます。ご留意ください。
おわりに
弊事務所はフェムテックの分野でビジネスしていこう、と考える事業者の皆さまを応援しています。
薬事規制にかかる一連の手続きについてはもちろん、規制上のどの製品カテゴリにするのが適切なのか、わからない!といったご相談もお受けしております。
最初の面談は無料ですので、企画段階からでも、手元に製品出来上がっていても、どうぞお気軽にご連絡ください。

2026年1月施行の改正行政書士法により、事業者の権利を守る伴走支援がさらに強化されました。詳しくは1/16公開予定のこちらの記事で。
参考
- "Product Classification Lubricant, Personal". U.S. Food and Drug Administration. Last Updated: 06/20/2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=4212. (参照2022−06−24)
- "Product Classification Lubricant, Personal, Gamete, Fertilization, And Embryo Compatible". U.S. Food and Drug Administration. Last Updated: 06/20/2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=4245. (参照2022−06−24)
- 独立行政法人医薬品医療機器総合機構. "一般的名称(検索) 歯科用潤滑材". 医療機器基準等情報提供ホームページ. (更新日不明). https://www.std.pmda.go.jp/scripts/stdDB/JMDN/stdDB_jmdn_resr.cgi?Sig=1&Select=1&jmdn_no=3618&kjn_no=0. (参照2022−06−24)
- ."FDA 510K Medical Device". National Personal Lubricant Association. (更新日不明). https://www.personallubricant.org/blank-page. (参照2022−06−24)
お気軽にご相談ください。
- 初回相談は無料です。
- 行政書士には秘密保持の義務が課せられております。
- フォームに入力されたメールアドレス以外に、当事務所から連絡差し上げることはいたしません。
“ラブローションを取り巻く規制” に対して1件のコメントがあります。
コメントは受け付けていません。