【化粧品ポイント解説】「56の効能効果」変遷史:規制緩和が製造販売業者に課した「品質保証の責任」

【化粧品ポイント解説】「56の効能効果」変遷史:規制緩和が製造販売業者に課した「品質保証の責任」
導入:なぜ今、効能の変遷を知るべきか—「自由」と引き換えに負った歴史的責任
化粧品の持つ可能性を広げ、市場に多様な製品を生み出す原動力となってきたのが、薬機法(旧薬事法)の変遷とともに変化してきた「効能効果の範囲」です。
特に2000年代以降の大きな規制緩和は、事業者に新製品投入の自由をもたらした反面、製品の品質と安全への最終責任を格段に重く課しました。この歴史的な転換点と、それに伴い確立された製造販売業者の責任体制(GQP・GVP)の意義を理解することは、現代の経営層・責任者の皆様にとって、法令遵守と事業の持続可能性を担保するための根幹となる「教訓」です。
本記事では、化粧品の効能の歴史的変遷を深く掘り下げ、それが現代の製造販売業者にどのように影響し、今、最も注意すべき広告規制のリスク管理に繋がるのかを解説申し上げます。
1. 効能の変遷に見る薬機法規制の潮流—「許可制」から「届出制」への転換が意味するもの
化粧品に関する法規制の歴史は、行政の管理体制から、企業の自己責任と自主管理へと、その重心を移してきた軌跡とも言えます。見ていってみましょう。
薬事法制定までの経緯
- 1873年(明治06年):「医制」公布、「薬剤取調ノ方法」
- 1943年(昭和18年):戦時中、複数にバラけていた法律をまとめ「薬事法(昭和18年3月12日法律第48号)」を制定
- 1948年(昭和23年):戦後、1943年薬事法を全面改訂した「薬事法(昭和23年7月29日法律第197号)」が制定
- 1960年(昭和35年):1948年薬事法を全面改訂した「薬事法(昭和35年法律第145号)」が制定
この1960年薬事法が、現在の薬機法に継承されていると言われています。ですので、ここから化粧品の効能の範囲の変遷を見ていこうと思います。
1960年(昭和35年)薬事法制定時:個別許可と類別効能の時代
この薬事法制定当初、化粧品の効能の範囲は製品の「類別ごと」に細かく分けられ、原則として厚生大臣の個別の許可が必要な「行政主導型」の管理体制でした。
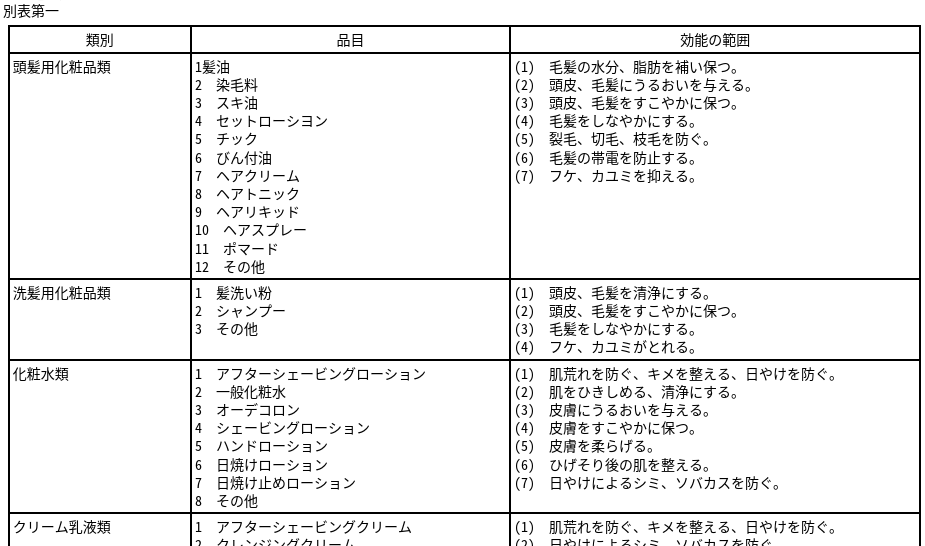
このときの化粧品の効能の範囲は、昭和36年2月8日付け薬発第44号薬務局長通知「薬事法の施行について」の別表第1にて示されています。
一部だけキャプチャーした画像を貼り付けますが、類別ごとに分けられており、現在の医薬部外品の効能効果の範囲っぽい表ですね。
2000年(平成12年)の激動:規制緩和と企業責任の転換
昭和61年から2000年までのあいだに、製品の許可制度は次のように段階的な変遷を果たしています。
- 個別許可制度(個別品目の製造許可)
- 種別許可制度(化粧品種別許可基準に合致すれば届出で済む)
- 種別承認制度(種別許可基準外の成分は承認範囲内であれば届出で済む)
中でも化粧品業界にとって大きな転換点となったのは、国を挙げての規制緩和計画の中で行われた2000年の大規模規制緩和です。この規制緩和は、平成7年(1995年)3月31日閣議決定の「規制緩和推進計画」や平成10年(1998年)3月31日閣議決定の「規制緩和推進3カ年計画」に基づき、「化粧品規制の在り方に関する検討会」で議論されました。
この規制緩和の流れにより、それまでの製品の許可制度を化粧品基準+届出制に変更したことで、新規参入や新製品投入が大幅に簡略化されました。化粧品戦国時代の幕開けとなったわけです。当然ながら企業責任は大きくなったのですけどね。ここあたりの改正は、平成12年9月29日付け医薬発第990号「化粧品規制緩和に係る薬事法施行規則の一部改正等について」で取りまとめてくれています。ありがたい!
このとき、化粧品の効能の範囲も改定され、平成12年12月28日付け医薬発第1339号(医薬安全局長通知)で、類別に効能を規定する現行の仕組みを廃止し、化粧品に該当する効能全体を規定する仕組み(55項目)に改められました。こちらも一部キャプチャー画像を。55項目あります。

この大規模規制緩和により、事前規制から事後チェックへの転換と、品質と安全に対する責任が全面的に事業者に委ねられる(自己責任)への転換、とがなされました。先に「企業責任は大きくなった」と書いてたのはこういうことですね。
2005年(平成17年)の製販分離
化粧品の効能の範囲の変動はありませんが、近年の薬事行政を語るうえではここは欠かせないポイントなのでちょっとだけ。
この規制緩和の流れは、2005年まで続きます。2005年の薬事法改正では「製造業」と「製造販売業」の製販分離が行われました。市場に対する最終責任を負う「製造販売業者」が法的に確立され、製造機能を持たない企業も参入しやすくなりました。また製造の外部委託や輸入もしやすくなりましたね。
製造販売業者には、GQP(品質管理基準)とGVP(製造販売後安全管理基準)の体制構築が義務付けられています。規制緩和により市場への参入が容易になった分、事業者は行政の代わりに、製品の設計から製造、流通、市販後に至るまでの全てのプロセスに対し、強固な自主管理体制を構築し、維持する責務を負うことになりました。
これから許可を取得しようと検討中の経営者様は、こちらの記事で体制構築の本質を深くご理解ください。
2011年(平成23年)~現在
そして化粧品の効能の範囲は、2011年に「(56) 乾燥による小ジワを目立たなくする。」が加わり、現在の56の範囲となりました。これは、平成23年7月21日付け薬食発0721第1号厚生労働省医薬食品局長通知「化粧品の効能の範囲の改正について」の別紙にて示されています。

2. 「56の効能効果」の遵守と「科学的根拠」の必要性—責任者が確保すべき品質保証の土台
さて、ここまで化粧品の効能の範囲の変遷について見てきました。
現在、化粧品が謳える効能効果は、先述の通り56項目の範囲内に限定されています。この「56の効能効果」は、化粧品の製造販売業者が理解しておくべき事項です。
効能の範囲は「製品の効能」と「広告の訴求範囲」の両方
ここで皆さん、ちょっと疑問に思ったことはないでしょうか。
化粧品の効能の範囲って、化粧品そのものの効能がその範囲に収まらなくてはならないということ?
それとも、化粧品の広告ではその範囲の効能しか訴求できないということ?
少なくとも私は実は、この点あやふやな感じ仕事してた時期があります。
これに関してですが、端的に言うと、その両方だと私は理解しています。だって通知でこう書いてるから。
「化粧品の効能として表示し、広告することができる事項については、局長通知別紙の別表第一に掲げる化粧品の効能の範囲とし、かつ当該製品について該当する効能の範囲であること。」
平成23年7月21日薬食審査発0721第1号、薬食監麻発0721第1号「化粧品の効能の範囲の改正に係る取扱いについて」
そういえば、平成12年の時点の通知でもこう書いてありましたしね。
「類別に効能を規定する現行の仕組みを廃止し、化粧品に該当する効能全体を規定する仕組みに改めることとした。」
平成12年12月28日医薬発第1339号「化粧品の効能の範囲の改正について」
56項目目の効能にみる「根拠主義」
2011年に追加された56項目目「乾燥による小ジワを目立たなくする。」の効能は、現代の化粧品ビジネスにおいて科学的根拠がいかに重要であるかを象徴しています。
この効能を標榜するためには、日本香粧品学会の「化粧品機能評価法ガイドライン」に基づき試験を行い、その効果を客観的に確認することが、日本化粧品工業連合会の自主基準等により求められています。別軸ですが、景品表示法の観点からも、標榜する効果については客観的なデータを保持しておくことは重要です。
製品の品質と安全性を科学的に保証するための試験検査の重要性については、こちらの記事で詳細を解説しています。
3. 効能の範囲を逸脱するリスク:経営層が認識すべき課徴金リスク—虚偽・誇大広告の教訓
規制緩和の歴史的教訓を現代に活かす上で、最も重大なリスクが広告規制の違反です。製造販売業者が謳う効能効果が56項目の範囲を超えた場合、それは薬機法第66条第1項の虚偽・誇大広告に該当する可能性があります。例えば、「皮膚を若返らせます」や「アンチエイジング」といった表現は、法第66条による禁止事項に該当します。
2021年導入の課徴金制度が変えたリスク
経営層の皆様が特に認識すべきは、課徴金制度です。これは、行政指導や業務停止命令に加え、違反事業者に対して売上額に一定の割合を乗じた金額が課される制度です。(※この制度の導入時期や割合(4.5%)については、本記事の論旨の根拠となる通知資料内では確認できませんでしたが、極めて重大なリスクであることは間違いありません。この点については、行政書士事務所の見解として、外部情報に基づき記載しています。)
この制度の下では、効能の範囲を逸脱した広告表現は、企業財務とブランド信用に直接的な打撃を与える、経営上の深刻なリスクへと変化しました。
課徴金制度の詳細と、今すぐできる対応策については、こちらの記事をご参照ください。
3-1. 【実務担当者への導線】効能の境界線を踏み外さないための具体的チェック
「効能の範囲」を正確に理解し、「広告の範囲」を混同しないこと。これは、製造販売業者の現場で最も判断が問われる課題です。
特に、「エイジングケア」の表現は、化粧品等に認められた効能・効果の範囲内で行う、年齢に応じた化粧品等によるお手入れ(ケア)として表現することは認められていますが、素肌の若返り効果があるかのような表現は不適切であるなど、判断が難しいケースが多々あります。
この判断ミスは、結果として、責任者である経営層に課徴金という形で跳ね返ってきます。具体的なNG表現と、法令の範囲内で誠実に製品の魅力を伝えるための言い換え方については、以下の専門記事で網羅的に解説しています。実務ご担当者様への周知徹底にお役立てください。
まとめ:信頼を築くための「三位一体」コンプライアンス—歴史的教訓をGQP/GVP体制に活かす
化粧品の「効能の範囲」の変遷は、規制の自由化が進んだ裏側で、企業が負うべき責任の重さが増したという歴史的な教訓を示しています。
市場の信頼を勝ち取り、事業を継続的に展開していくためには、以下の「三位一体」のコンプライアンス体制が不可欠です。
- 適法な効能の正確な理解(56項目の厳守)
- 厳格な品質管理体制の維持(GQP・GVPの運用)
- 適正な広告表現の徹底(薬機法・景表法の遵守)
効能の範囲を逸脱しない広告戦略は、まさしく強固なGQP・GVP体制の適切な運用から生まれるものです。既存のGQP・GVP体制が、この歴史的転換が課した重い責任に見合った実効性を確保できているか、定期的な見直しを行うことが重要です。
GQP・GVP体制の運用見直しについては、こちらの記事もぜひ見ていただきたい記事です。
効能・広告表現のリーガルチェック:リスクを回避し、市場の信頼を勝ち取るために
効能の範囲の理解不足や、広告表現の微妙な判断ミスは、課徴金制度の下では致命的なリスクとなり得ます。製品の市場投入前、または大規模なプロモーション実施前に、専門家による客観的な視点を取り入れることは、リスクヘッジのための必須の投資と言えます。
おおぐし行政書士事務所では、薬機法・景表法に精通した行政書士が、貴社の化粧品広告表現が「56の効能効果」の範囲内で適法かつ最大限の魅力を伝えるものとなっているか、徹底したリーガルチェックを提供申し上げます。
貴社の広告表現は、法的な観点から見ても妥当でしょうか?
ウェブサイトのお問い合わせフォームより、リーガルチェックのご相談を承ります。お問い合わせをお待ち申し上げます。
お気軽にご相談ください。
- 初回相談は無料です。
- 行政書士には秘密保持の義務が課せられております。
- フォームに入力されたメールアドレス以外に、当事務所から連絡差し上げることはいたしません。