【2026年最新】アルコール消毒液の販売・保管に関わる「4つの法律」基礎講座|特例廃止後の完全ガイド
行政書士の大串です。
コロナ禍を経て、オフィスや店舗、家庭における「衛生インフラ」として定着したアルコール消毒液。 かつては需給ひっ迫に対応するため柔軟な対応が取られていましたが、2024年(令和6年)6月の「高濃度エタノール製品」特例の完全廃止(参考1)により、現在は平時の厳格な法規制へと完全に回帰しています。
「まだ特例の表示のまま販売している」「物品用の消毒剤は雑貨扱いでいいはず」
その認識は、現在では重大なコンプライアンスリスクとなり得ます。 特に、異業種からの参入をご検討中の事業者様や、企業の総務・管財担当者様においては、最新のルールの把握が急務です。
本記事では、消毒用エタノールビジネスを縛る「4つの法律(消防法、酒税法、アルコール事業法、薬機法)」に加え、近年の法改正で重要度が増した「労働安全衛生法(化学物質管理)」や、薬機法の新たな枠組みとして制度化された「物品用医薬部外品」について、2026年時点の最新情報に基づき解説します。
エタノール製品にかかる規制
エタノール製品にかかる規制は多重構造になっています。ざっくりまとめてみました(参考2及び3)。 実際はこの図を頭においておいて、取り扱う製品ごとに規制の範囲か否かを個別確認していきます。
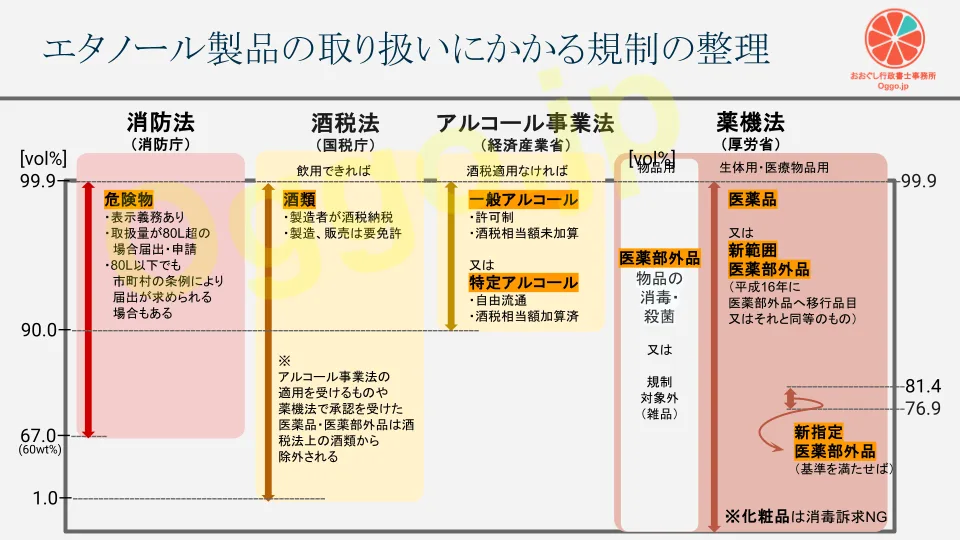
- 消防法(消防庁)における危険物
- 酒税法(国税庁)における酒類
- アルコール事業法(経済産業省)における一般アルコール又は特定アルコール
- 薬機法(厚労省)における以下の区分
- 医薬品
- 医薬部外品(新範囲:殺菌消毒薬/新指定:外皮消毒剤)
- 物品用医薬部外品(物品の消毒・殺菌目的) ※旧来の「雑品」から移行
- 規制対象外の雑品(「除菌」等の表現にとどまるもの)
【人体用】消毒目的の場合はどの選択肢を選ぶか
繰り返しになりますが、大前提として「コロナ禍の特例措置」は完全に終了しています。
1. 特例終了と「未承認医薬品」のリスク(薬機法第68条)
コロナ禍において、高濃度エタノール製品(雑貨)を手指消毒用として転用することを認めていた特例措置は、2024年(令和6年)6月30日をもって完全に終了しました(参考1)。
現在、医薬品または医薬部外品の承認を受けていない製品(雑貨)について、「手指消毒」や「消毒用エタノールの代替」といった効能効果を謳って販売することは、薬機法第68条(未承認医薬品の広告禁止)違反となります。 パッケージや広告で「消毒」を謳うことはもちろん、文脈から消毒を暗示させる表現(手指のイラストと洗浄イメージの併記など)も指導対象となるリスクが極めて高まっています。
2. 人体用の正攻法:新指定医薬部外品(外皮消毒剤)
人体に使用する目的の消毒剤は、医薬品又は医薬部外品として承認を得る必要があります。
化粧品では殺菌、消毒は訴求できません。 これから参入する場合、難易度の高い「医薬品」よりも、要件の明確な「医薬部外品」を目指すのが手堅い戦略です。
医薬部外品としての選択肢は以下の2つです。
(1) 新指定医薬部外品(外皮消毒剤)
- 概要:厚生労働大臣が指定する医薬部外品のうち、「(15)すり傷、切り傷、さし傷、かき傷、靴ずれ、創傷面等の消毒又は保護に使用されることが目的とされている物」に該当するものです。
- 基準: 「外皮消毒剤 製造(輸入)承認基準」に適合すること(エタノール濃度 76.9〜81.4vol% 等)。
- メリット: 基準が明確で、人体用消毒ビジネスの「王道」と言える選択肢です。
(2) 新範囲医薬部外品(殺菌消毒薬)
- 概要:厚生労働大臣が指定する医薬部外品のうち、「(9)殺菌消毒薬」に該当するものです。
- 基準: 既承認品目と「有効成分の分量」「効能効果」「用法用量」等が同一であること。
- 難易度: 既承認情報の調査や同等性の証明が必要で、難易度は高めです。
【物品用】「物品用医薬部外品」の新設と機会
従来、物品(テーブルやドアノブ等)の消毒剤は「雑貨」扱いでしたが、2023年より「物品の消毒・殺菌」を目的とした製品も、正式に医薬部外品として認められるようになりました(資料4)。
これにより、雑貨では謳えなかった「消毒」「殺菌」という強力な訴求が可能になりました。
- 定義:物品の消毒・殺菌の用に供されることが目的とされているもの
- 対象: 家具・器具・物品、室内、浴室、便所、哺乳びん、食器 等
- メリット: 「除菌(雑貨)」ではなく「消毒(医薬部外品)」として明確な差別化(参入障壁)が可能。
消防法(危険物):意外と低い「80L」の壁に注意
消毒用エタノール(濃度60wt%以上)は、消防法上の「危険物 第四類(引火性液体)のアルコール類」に該当します(資料5)。 ここで重要になるのが、保管量に応じた「指定数量」の規制です。
「指定数量(400L)」だけを気にしていませんか?
アルコール類の指定数量は400Lですが、その5分の1である「80L(指定数量の0.2倍)」を超えた時点で、消防法に基づく「少量危険物」としての規制対象となります。
- 80Lとは?: 一般的な一斗缶(18L)で換算すると、わずか4.4缶です。
- 500mlボトルなら: 160本分です。
| 保管数量(総量) | 該当区分 | 必要な法的対応 |
|---|---|---|
| 80L未満 | 規制対象外 | 特になし(ただし火気厳禁等の一般的注意義務あり) |
| 80L以上 400L未満 | 少量危険物 | 管轄消防署への「少量危険物貯蔵取扱所」の届出をして、検査を受ける必要あり。消火設備の設置等が義務付けられる。 |
| 400L以上 | 指定数量以上 | 「危険物貯蔵所」としての設置許可が必要。建屋の耐火構造化、保安距離の確保など、極めて厳格な基準が適用される。 |
オフィスの備蓄や店舗のバックヤードでも容易に超えてしまう量です。 80L以上を保管・貯蔵する場合、所轄の消防署への届出や、保管場所の構造・設備基準(不燃材料の使用など)の遵守が求められます。
コロナ禍の緊急対応時には、各消防本部で運用上の特例(届出の緩和等)が見られましたが、現在は平時の運用に戻っています。「知らずに届出漏れ」とならないよう、在庫量の管理を徹底してください。
その他市町村の火災予防条例により、消防法とは別に基準が定めている場合があります。

酒税法・アルコール事業法:「餅は餅屋」への回帰
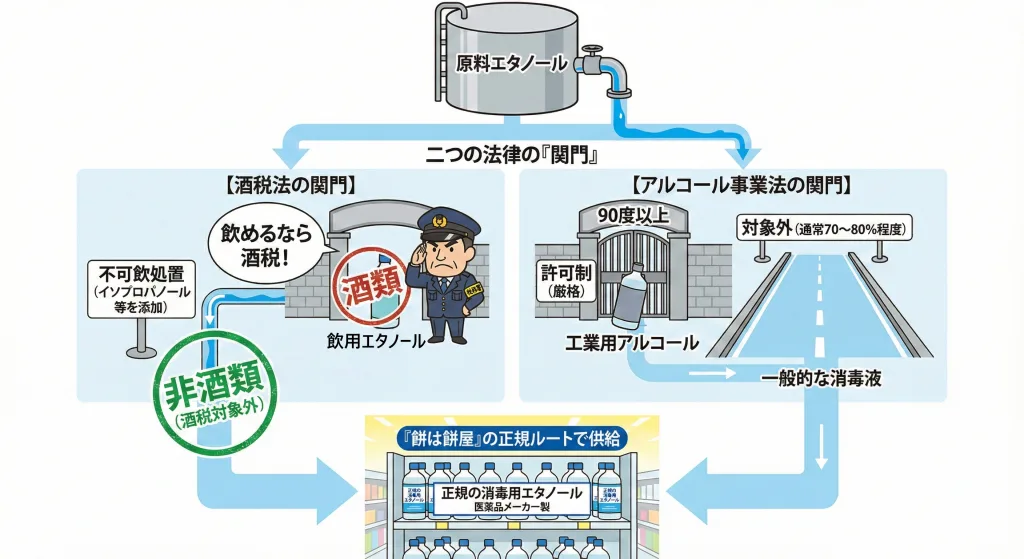
酒税法:飲用不可処置が基本
酒税法では、アルコール度数1度以上の液体は「酒類」とみなされ、酒税が課されます。 消毒用エタノールが高額にならないよう、通常は「不可飲処置(イソプロパノール等を添加して飲めなくする)」を施すことで、酒税法の対象外(非酒類)として流通させます。 コロナ禍では、酒造メーカーが高濃度エタノール(酒類)を消毒用代替品として免税販売するケース(例:獺祭エタノール(資料6)等)もありましたが、特例終了に伴い、こうした製品は市場から姿を消しつつあります。
アルコール事業法:工業用は90度以上から
アルコール分90vol%以上のものは「工業用アルコール」として、経済産業省の管轄(アルコール事業法)に入ります。 製造・輸入・販売・使用には許可が必要ですが、消毒用エタノール(通常70〜80%程度)であれば、この法律の規制対象外となるケースが一般的です。
現在は、医薬品・医薬部外品の製造販売業許可を持つ「医薬品メーカー(餅は餅屋)」による、正規の消毒剤供給体制が完全に復旧しています。
見落としがちな第5の法律:労働安全衛生法
2024年4月より、化学物質の「自律的管理」に関する規制が本格施行され(資料7)、エタノールは「リスクアセスメント対象物」に指定されています。 これは、消毒用エタノールを製造・販売する側だけでなく、業務で使用するユーザー(事業者)側にも新たな義務が発生することを意味します。
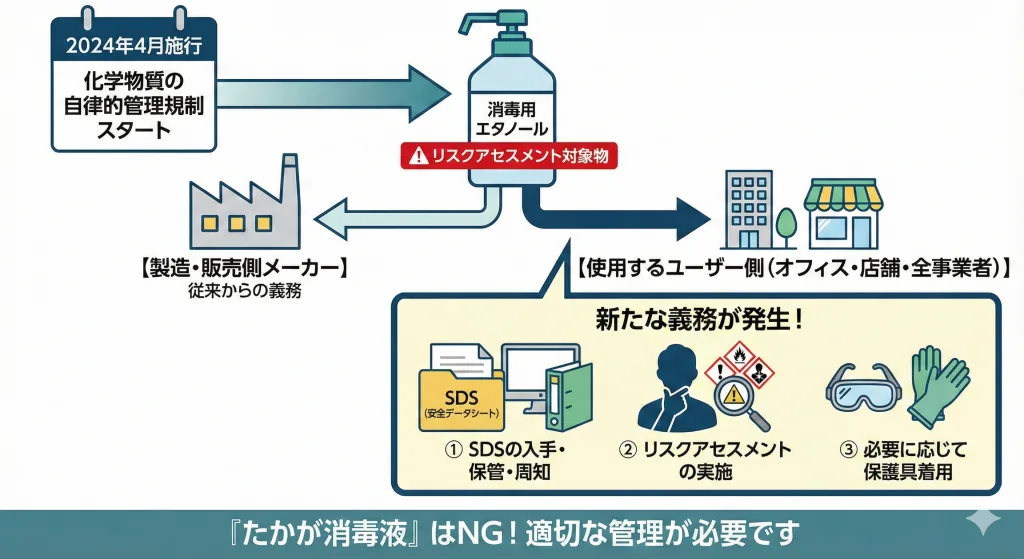
事業者に求められる対応
消毒用エタノールを事業所で使用する場合、以下の対応が必要です。
- SDS(安全データシート)の入手と保管 メーカーからSDSを取り寄せ、作業者がいつでも見られるように保管・周知する必要があります。
- リスクアセスメントの実施 その化学物質(エタノール)を使うことで、労働者にどのような危険があるかを特定し、リスクを見積もる必要があります。
- 保護具の着用 リスクアセスメントの結果に基づき、必要に応じて保護メガネや手袋等の使用を徹底します。
「たかが消毒液」と思わず、労働安全衛生法上の「化学物質」として適切に管理することが求められます。
こちらについて詳しくは、顧問社労士さんにご確認くださいね。
おわりに
アルコール消毒ビジネスは、特例終了により『プロの領域』に戻りました。これから参入を考える方、あるいは既存製品の法規制対応(規格改正等)に不安がある方は、当事務所へ直接ご相談ください。
医薬部外品にかかる一連の手続きについてはもちろん、医薬部外品にするしないの判断がつけられない!といったご相談もお受けしております。 最初の面談は無料ですので、企画段階からでも、手元に製品出来上がっていても、どうぞお気軽にご連絡ください。
参考
- "新型コロナウイルス感染症の発生に伴う消毒用エタノール関連事務連絡の廃止について". 厚生労働省. (参照2026-01-06)
- "Q1 酒類の定義を教えてください。". 国税庁.更新日不明. https://www.nta.go.jp/taxes/sake/qa/01/01.htm. (参照2022−06−17)
- "アルコール事業法の理解を深める". 経済産業省製造産業局アルコール室. 平成26年3月. https://www.meti.go.jp/policy/alcohol/alc_pamphlet_rev.pdf. (参照2022−06−17)
- 令和5年4月28日厚生労働省告示第181号
- "消毒用アルコールの貯蔵に係る運用について". 東京消防庁. 更新日不明.https://www.tfd.metro.tokyo.lg.jp/lfe/office_adv/chozou.html(参照2026-01-06)
- 獺祭エタノール販売終了のお知らせ, 1月 6, 2026にアクセス、 https://dassai.com/news/info/005698.html
- 化学物質による労働災害防止のための新たな規制について|厚生労働省. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000099121_00005.html(参照2026-01-06)
お気軽にご相談ください。
- 初回相談は無料です。
- 行政書士には秘密保持の義務が課せられております。
- フォームに入力されたメールアドレス以外に、当事務所から連絡差し上げることはいたしません。
“【2026年最新】アルコール消毒液の販売・保管に関わる「4つの法律」基礎講座|特例廃止後の完全ガイド” に対して2件のコメントがあります。
コメントは受け付けていません。